SAFER Wearables Study

The SAFER Wearables Study: A study of the acceptability and performance of wearables for atrial fibrillation screening in older adults
This page provides details of the study and links to study resources:
Overview of the Study
Overview of the Study
Background
Atrial fibrillation (AF) is an irregular heart rhythm which causes a five-fold increase in the risk of stroke. Approximately one in ten people aged over 70 have AF. If AF is recognised then the risk of stroke can be reduced by taking tablets regularly. AF can be difficult to recognise as it can occur without symptoms and only intermittently. Consequently, AF is not recognised in many people, meaning they live with an increased risk of stroke. Therefore, it is important to find ways to identify AF more reliably.
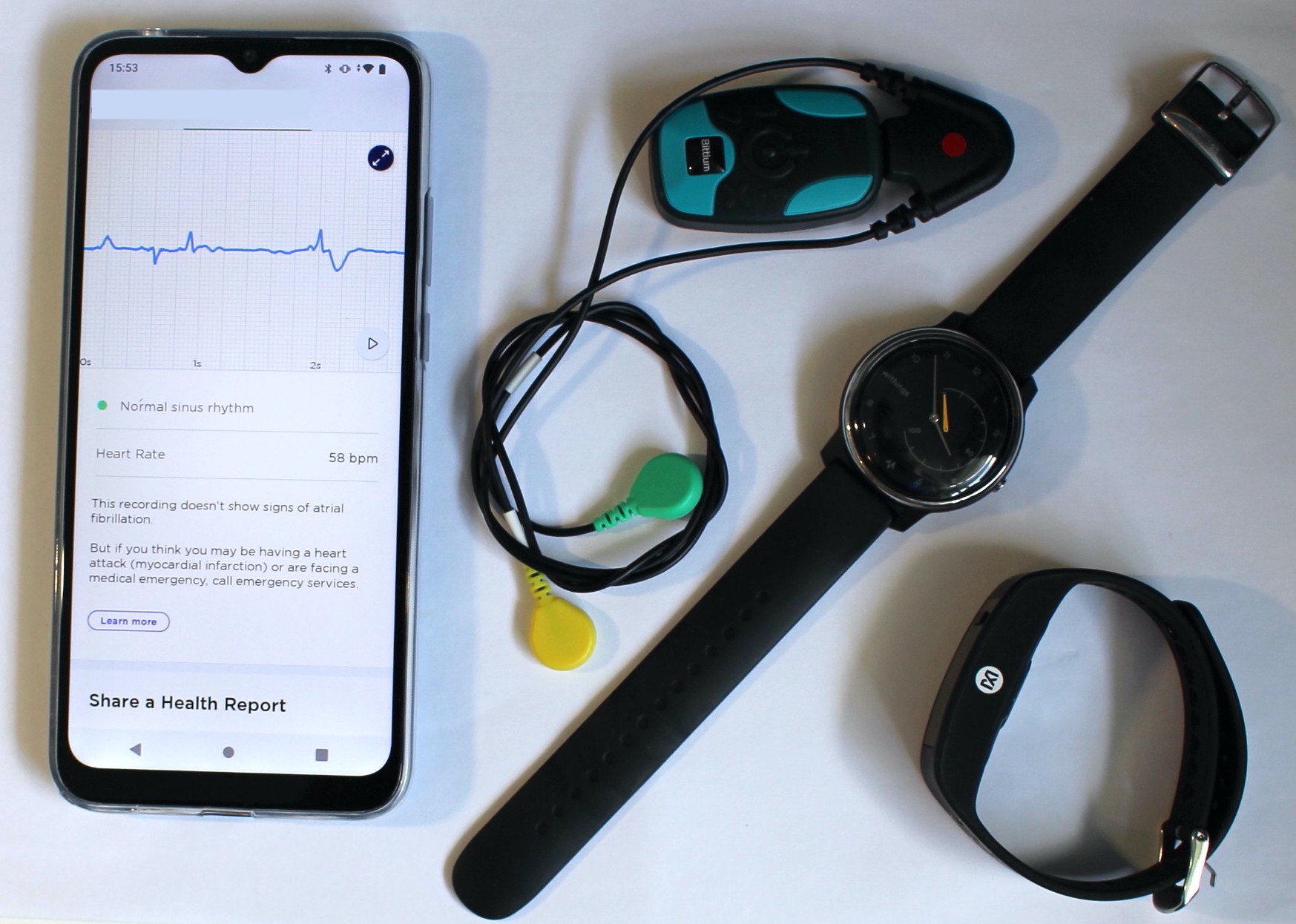
Recently, wearable devices have been developed which could be useful for identifying AF. Several devices can monitor heart activity in daily life, including wristbands, smart watches and chest patch monitors. The aim of this study is to assess the acceptability and performance of wearables for use in AF screening in older adults.
Aim
The primary objective is to determine the feasibility of measuring inter-beat-intervals using a wristband.
The secondary objectives are:
- To determine the acceptability of wearables,
- To determine the acceptability of the screening approach,
- To assess the performance of wearables for acquiring signals,
- To assess the performance of signal processing algorithms,
- To assess the performance of wearables for AF screening.
Who can participate?
Selected people who have previously participated in the SAFER Programme can participate in this study. The Investigators (at the University of Cambridge) will invite previous SAFER Programme participants to also participate in this study, aiming to enrol 65 without AF, and 65 with AF.
What does the study involve?
Participants will be asked to wear three devices for seven days: Two wristbands (like watches), and one chest patch (like a plaster). These devices will collect measurements of their heart’s activity. The Investigators will also ask participants to tell them how they found wearing the devices by completing a questionnaire. The Investigators will compare how participants found wearing each device, and how accurately each device identifies AF.
Further Information
Further information on the study can be found at ClinicalTrials.gov (NCT04715555).
Study Planning
Study Planning
Planning
Notebooks detailing the planning for the study are available here. In particular, the following notebooks may be of interest:
Equipment Testing
Details of equipment testing are provided in the following posts:
Equipment testing is ongoing.
Standard Operating Procedures (SOPs)
SOPs providing fine detail on the practicalities of carrying out the study are available here.
Study Methodology
Study Methodology
Ethics Approval
Details of the ethical approvals for the study are available here.
Documentation
Study documentation will be made openly available in an online repository in the future.